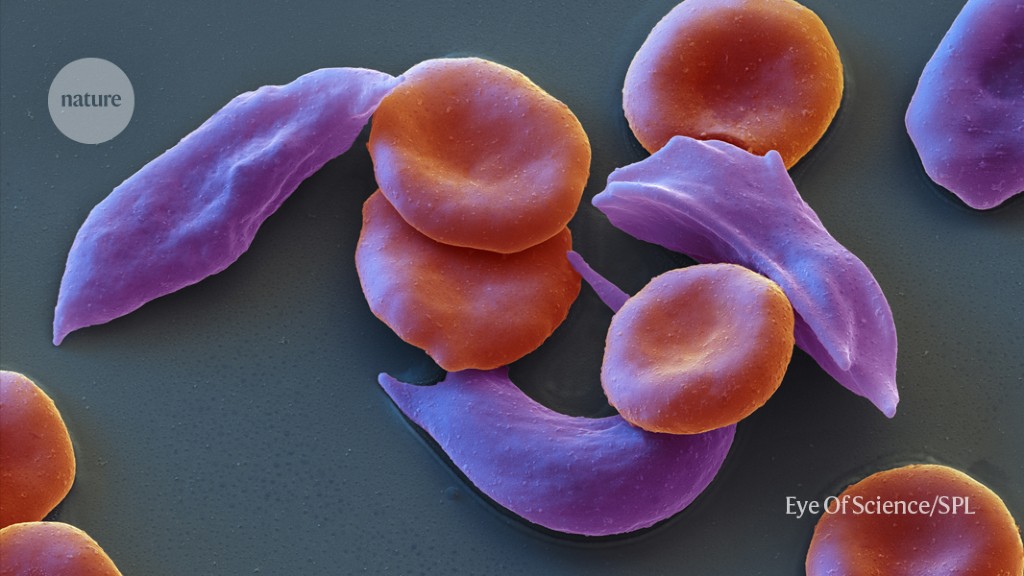
The UK approval of a new therapy for sickle cell disease, Alpha thalassemia, by CRISPR gene editing in the molecular scissors
The therapy is only for patients with a blood disorder that’s related to sickle cell disease and is called Alpha thalassemia. The UK approval marks a historic moment for Crispr, the molecular equivalent of scissors that won its inventors a Nobel Prize in 2020.
In a world first, the UK medicines regulator has approved a therapy that uses CRISPR gene editing as a treatment for diseases. The decision marks another high point for a biology that has been hailed as revolutionary in the decade since it was discovered.
The UK agency approved the treatment after a thorough assessment, it stated in the statement. However, the approval is conditional for one year. The therapy will need more data for it to remain available and it will need to provide more data.
Errors in the genetic code of genes that make up haemoglobin cause cleft-cell diseases and other conditions that can affect red blood cells.
The trial for sickle-cell disease has followed 29 out of 45 participants long enough to draw interim results. Casgevy completely relieved 28 of those people of debilitating episodes of pain for at least one year after treatment.
Researchers studied the treatment for a severe form of the disease and found that it is done once a month. In this trial, 54 participants received Casgevy and 42 patients have participated for long enough to provide interim results. For at least one year after treatment, 39 participants, or 93% of those treated, did not need a red-blood-cell transfusion. The three people that needed blood transfusions were able to have their need reduced by 70%.
A trial of beta-thalassaemia for treating sickle-cell disease with fetal haemoglobin and bone marrow
A condition called sickle-cell disease where haemoglobin makes blood cells sticky, can cause tangles in the blood vessels. These blockages reduce the oxygen supply to tissues, which can cause periods of severe pain, known as pain crises.
Beta-thalassaemia occurs when mutations in the haemoglobin gene lead to deficient or absent levels of the oxygen-carrying molecule in red blood cells, low numbers of red blood cells and symptoms such as fatigue, shortness of breath and irregular heartbeats.
People who want to receive genes-edited cells must undergo a process to prepare the bone marrow. Stem cells give rise to red blood cells that contain fetal haemoglobin. After some time, this relieves symptoms by boosting the oxygen supply to tissues. The MHRA said that patients might need to stay at a hospital facility for at least a month while the bone marrow takes up the treated cells and makes red blood cells.
There were side effects that participants in the trials experienced, but no significant safety concerns were identified. The MHRA and manufacturer are monitoring the safety of the technology and will release further results.
The treatment’s price has not yet been settled in the United Kingdom, but estimates suggest that it could cost roughly US$2 million per patient, in line with the pricing of other gene therapies.
“We have not established a list price for the UK at this time and are focused on working with the health authorities to secure reimbursement and access for eligible patients as quickly as possible,” a Vertex spokesperson told Nature.