PFL3: A Neuron Model for Control of Central Steering in Wild-type Fly Detectors using Neurotransmitters
MCFO, w[+t 7.7] W[+mC] of R57C10-FLPG5
flies were raised in an incubator on a light:dark cycle of 25 C at 50%- 70% relative humidity, and on a corn meal. The experimenters weren’t blinded to the genotype. For iontophoresis stimulus experiments (Fig. 2a,b) flies were grouped for analysis based on genotype. The sample sizes were decided based on convention in our field for standard sample sizes, as well as given published results and pilot data. All experiments used flies with at least one wild-type copy of the white (w) gene. Genotypes used in each figure are as follows.
pfl2 and pfl3 calcium images.
where (W) denotes an array of synaptic weights and (f) represents a nonlinear activation function (see below). We define PFL3R cells as the members of the PFL3 cell class that project their axons to the right hemisphere; PFL3L cells are the members of the PFL3 cell class that project their axons to the left hemisphere. Some past work on PFL3 cells had a different method for dividing them as compared to this one.
Our model is similar to several other models of central steering control. These studies, in turn, built upon the existing idea that vectors should be represented as sinusoidal spatial patterns of neural activity, so that vector addition can be implemented via the addition of sinusoids9,15,16,53,54. This idea of how rotational velocity commands can be generated using the right–left shift of the basis vectors9 was extended by Webb and colleagues. Our model takes advantage of new information from the automatic assignment of neurotransmitters50, as well as our neuroscience experiments. It is a different model in a few important ways. Most notably, our model shows how this network can adaptively control steering gain based on the magnitude of directional error (via PFL2 cells). PFL2 regulators have been proposed as a non-steering-related role in previous studies. Our model shows how the steering system can be avoided by the cells when error is low by boosting steering only if error is high, and by slowing down steering when error is high.
The goal input will have no influence on the right– left difference in PFL3 if the activation function (f) is nonlinear. We use S = 1 by default, except in Figs. We are looking at the effect of lowering S.
The neural representation of the goal direction as a sinusoidal pattern over neural space and its implementation in the PFL3L, L and L cells
$$\begin{array}{l}\,\,{\rm{PFL}}2\,=\,f(S\times (\cos (\theta -{\theta }{0}-{\bf{h}}+18{0}^{\circ })+A\times \,\cos ({\theta }{g}-{\theta }{0}-{\bf{h}})))\ {\rm{PFL}}3{\rm{R}}\,=\,f(S\times (\cos (\theta -{\theta }{0}-{\bf{h}}+67.{5}^{\circ })+A\times \,\cos ({\theta }{g}-{\theta }{0}-{\bf{h}})))\ {\rm{PFL}}3{\rm{L}}\,=\,f(S\times (\cos (\theta -{\theta }{0}-{\bf{h}}-67.{5}^{\circ })+A\times \,\cos ({\theta }{g}-{\theta }_{0}-{\bf{h}})))\end{array}$$
We model the neural representation of the goal direction (θg) as another sinusoidal pattern over neural space, which is reasonable, because the goal direction can be thought of as just a special head direction, and head direction is represented as a sinusoid. The goals of the PFL2 and PFL3R cells are the same, and so we assume this is the case for the PFL3L cells. The goal representation will change if the offset of the head direction system is changed. The peak activity in goal cells will move left as the goal direction rotates right. The model implementations that we use measure the goal signal as if it were the same as the head direction signal, but some of the results could potentially be explained by a mechanism that modifies A.
In the analysis described in the previous paragraph, we used glomerular angles implied by the Δ7 innervation of the protocerebral bridge (Extended Data Fig. 5e–g Alternatively, we could have used glomerular angles based on the innervation of EPG neurons (Extended Data Fig. 5c). We decided to use the 7 scheme because the majority of the PFL3 neuron’s synaptic input is provided by 7 neurons. 5b).
The hemibrain connectome: a new model of steering signals based on the right-left difference in the activity of descending neurons
$$\begin{array}{c}{\rm{DNa}}02{\rm{R}}=f(\Sigma {W}{{\rm{DNa}}02{\rm{R}},{\rm{PFL}}3{{\rm{R}}}{j}}\times {\rm{PFL}}3{{\rm{R}}}{j}+\Sigma {W}{{\rm{DNa}}02{\rm{R}},{\rm{DNa}}03{\rm{R}}}\times {\rm{DNa}}03{\rm{R}})\ \ {\rm{DNa}}02{\rm{L}}=f(\Sigma {W}{{\rm{DNa}}02{\rm{L}},{\rm{PFL}}3{{\rm{L}}}{j}}\times {\rm{PFL}}3{{\rm{L}}}{j}+\Sigma {W}{{\rm{DNa}}02{\rm{L}},{\rm{DNa}}03{\rm{L}}}\times {\rm{DNa}}03{\rm{L}})\end{array}$$
The model is meant to understand how steering signals arise from the head direction system We take the steering signal as the right–left difference in the activity of DNa02 descending neurons, because these neurons have been shown to predict and influence steering4:
The hemibrain connectome data was obtained from the website neuprint.janelia.org and used to perform a analysis of the data using the neuprintr natverse 1.1 software package.
There are 12 complete PFL2 cells, 13 complete PFL3 cells and one nearly complete DNa03 cell in the hemibrain connectome, with over 100 presynapses associated with each of these cells. In the hemibrain dataset, there are still many images of the presynaptic sites in this cell even though the axon terminal is not present. 12 of 12 PFL2 neurons, 13 of 13 PFL3 neurons and one of 1 DNa03 neuron were predicted with a recent algorithm50,51. This algorithm predicts transmitters on a per-synapse basis, with an error rate that varies with cell and transmitter type. For PFL2 and PFL3 neurons, 74% of high-confidence presynapses (confidence score greater than or equal to 0.5) are predicted as cholinergic; the second most commonly predicted transmitter is glutamate (11%). For DNa03, 85.2 % of high-confidence presynapses are predicted as cholinergic; the second most commonly predicted neurotransmitter is glutamate (5.6%). This algorithm used 3,094 hemibrain neurons in its ground-truth data to train the model and included ground truth neurons identified as cholinergic using light microscopy pipelines and antibody staining or RNA sequencing. Among this ground-truth population, 73% of presynapses are correctly predicted as cholinergic. Predicting the sphinx is available from ref. 51.
This analysis assumes that g does not change much over the course of a trial. If θg did change dramatically, this would result in a lower ρ value for the trial and possibly also a reduced bump amplitude range value, despite the fly potentially being in a state of high goal fixation strength for the entire trial. The same could be said for fly that switched between periods of strong and weak goal fixation. The limitations of the analysis are shown in fig. 5k should, if anything, reduce our ability to detect a relationship between PFL2 activity and behaviour.
For Fig. 5e–g and Extended Data Fig. 7a–d, jumps were categorized as either corrected or uncorrected as described previously (see section ‘Classifying jumps as “corrected, high ρ” versus “uncorrected, low ρ”’). For each jump, the difference between the mean membrane potentials calculated over the 1 s before and following each jump was found, and the distribution of these values is shown for both categories in Fig. 5f,g. A two sample Brown–Forsythe test was used to determine whether the variance of membrane potential changes was significantly different between the two categories.
Detected IPSP frequency was calculated for the 5 s before or after the cue jump. The change in frequency before jump versus after jump was then compared to change in head direction relative to the cell’s preferred head direction produced by the cue jump. The answer was found after looking at the differences between the head direction before the jump and the cell preferred direction after the jump. The precue jump value was subtracted from the postcue jump value. A negative value indicates that the head direction was not close to the cell’s preferred head direction, and a positive value shows that the cell’s preferred head direction increased after the jump. The distance from the cell’s preferred head direction for each jump was plotted against the change in IPSP Frequency. MATLAB’s polyfit and polyval functions were used to find the line of best fit for the relationship between the two variables, while the corrcoef function was used to find the correlation coefficients of the relationship. Additionally, we used an unbalanced two-factor ANOVA to determine the significance of the relationship between change in frequency and change in head direction compared to that with cell identity.
To detect IPSPs we focused only on jump trials where the fly was immobile, so that we don’t have confounds associated with the fluctuations in the cells that are associated with movement transitions. After the potential was removed from the trace, the smoothing function was used to smooth it out using the loess method. We then calculated the derivative of the membrane potential (gradient function in MATLAB) and found local minima corresponding to periods of rapid decreases in membrane potential (findpeaks function in MATLAB, peak distance of 20 ms, threshold determined for each cell). We also generated a detrended version of the membrane potential by subtracting the median filtered membrane potential (500 ms window) and found local minima (findpeaks function in MATLAB, peak distance of 20 ms, threshold determined for each cell). We categorized IPSPs as indices where a negative peak was detected from the derivative of the membrane potential trace within 30 ms before a negative peak in the baseline corrected trace.
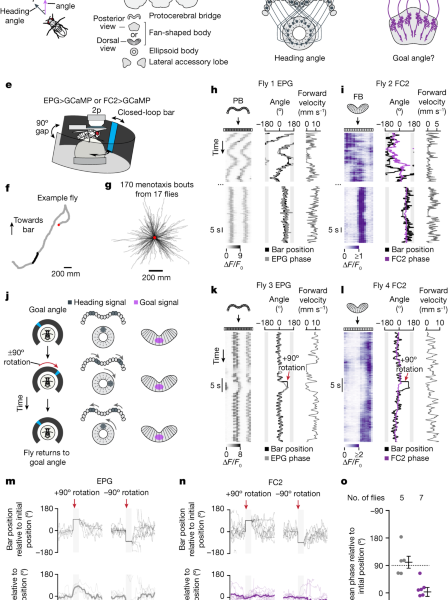
To track the phase and amplitude of PFL2 activity. 2c–g and 5j,k and Extended Data Figs. 3, 5 and 8, a sinusoid was fit independently to each time point of the z-scored ΔF/F activity across fan-shaped body and protocerebral bridge imaging trials:
For the head direction tuning analysis shown in Fig. We used a threshold of but only used data from segments where it was 0.7. We lowered the threshold on ρ for this analysis because we needed to include a larger number of time points in the analysis, to improve the resolution for binning the activity of cells into groups defined by θp − θg.
To obtain a continuous estimate of the stability of the flies’ heading angle (Extended Data Fig. 3o,p), we computed the flies’ mean heading vector length (R) similarly as described previously11. In brief, each heading sample point was treated as a unit vector. Each timepoint was assigned a value of R by the mean of the heading vectors that sat within a 60 or 120 s window. When calculating the value of R, time points in which flies were standing still were first omitted, since this would increase the value of R for trivial reasons.
Online ball tracking resampled to 60 Hz for accuracy alignment and vehicular analysis of a spherical treadmill
$${\rho }{t}=\sqrt{{\left(\frac{\Sigma \cos ({\theta }{{\rm{w}}})}{{N}{{\rm{w}}}}\right)}^{2}+{\left(\frac{\Sigma \sin ({\theta }{{\rm{w}}})}{{N}_{{\rm{w}}}}\right)}^{2}}$$
The end of each stack on the same card as online ball tracking signals was obtained by our software during calcium scans. These imaging time points were then resampled to the ball-kinematic data update rate of 60 Hz, allowing us to align the acquired volumes. Electrophysiology data were collected on the same acquisition card as online ball tracking signals, so alignment was not required; however, ball-tracking data were resampled to 1 kHz to match the sampling rate of the electrophysiology data.
The position of the spherical treadmill was computed online using machine vision software (Fictrac v.2.1) and output as a voltage signal for acquisition. For post hoc analysis, the voltage signal was converted into radians and unwrapped. Signals were then low-pass filtered using a second-order Butterworth filter with 0.003 corner frequency and downsampled to half the ball-tracking update rate. The function used to calculate the vehicality was from the MATLAB platform. The large velocity values were adjusted to 20 rad s1, and the time series was smoothed using the smooth function in MATLAB, and resampling the ball. Forward and sideways velocities were then converted to millimetres per second while yaw (rotational) velocity was converted to degrees per second.
Source: Transforming a head direction signal into a goal-oriented steering command
Low redshift PFL2 split-Gal4 GCaMP flies with Rigid motion correction in the x,y and z axes
$${y}{i}=\frac{{x}{i}-\,\underline{X}}{{\rm{MAD}}},{\rm{where}}\,\underline{X}={\rm{median}}\,{\rm{of}}\,X,\,{\rm{MAD}}={\rm{median}}\,(| {x}_{i}-\underline{X}| )$$
Analysis was performed in either MATLAB 2019 or MATLAB R2021a. There were 23 flies expressing GCaMP and 33 flies expressing GCaMP under the control of the PFL2 split-Gal4 line. Rigid motion correction in the x, y and z axes was performed for each trial using the NoRMCorre algorithm49. Each region of interest (ROI) was defined across the z-stack. For each ROI ΔF/F was calculated with the baseline fluorescence (F) defined as the mean of the bottom 10% of fluorescence values in a given trial (600 s in length). The normalized difference between the median and the modifiedz-score was used to calculate the modifiedz-score.
Post-incubation preparation and imaging of goat and goat anti-mouse brains for the MCFO experiments. Secondary antibody solutions and protocol-specific treatment
The primary solution for MCFO 48 contained anti-Bruchpilot and rat anti-Flag. NBP1-06712B) and rabbit anti-HA (1:300, Cell Signaling Technology, catalogue no. 3724S). The secondary antibody solution contained Alexa Fluor 488 goat anti-rabbit (1:250, Invitrogen, catalogue no. ATTO 666 goat anti-rat, Rockland catalog no. A1039 Both of those names, as well as the other one, were done by Alexa Fluor. goat anti-mouse is contained in the Invitrogen catalogue. A31553). DyLight 550 mouse anti-V5 is contained in the tertiary antibody solution. The item is called “MCA1360D555GA”.
The brains were put in 4% paraformaldehyde after being put in external saline for 1–3 days. Brains were washed with PBS before adding a blocking solution containing 5% normal goat serum (Sigma-Aldrich, catalogue no. G 9023). in PBS with 0.44% Triton-X (Sigma-Aldrich, catalogue no. T8787) For 20 minutes. The brain was washed in PBS and then isolated from the rest of the brain with a blocking solution for 24 h at room temperature. Primary and secondary antibodies were protocol-specific (see below). Brains were then rinsed with PBS and mounted in antifade mounting medium (Vectashield, Vector Laboratories, catalogue no. H-1000) for imaging. The MCFO protocols had a tertiary incubatee step about 24 h at the room temperature before mounting. Mounted brains were imaged using a 40, 1.15 NA oil-immersion objective. There were 200 to 500 x 200 image stacks at a depth of 1 m. Image resolution was 1,024 × 1,024 pixels. The primary antibodies for visualization of Gal4 expression patterns contained chicken anti-GFP. ab13970) and mouse anti-Bruchpilot (1:30, Developmental Studies Hybridoma Bank, nc82). The secondary antibody solution contained Alexa Fluor 488 goat anti-chicken (1:250, Invitrogen, catalogue no. A11039) and Alexa Fluor 633 goat anti-mouse (1:250, Invitrogen, catalogue no. A 20000. After whole-cell patch-clamp recordings, cell fills are visualized. streptavidin::Alexa Fluor 568 (Invitrogen, catalogue no. S11226) was added to the secondary and primary solutions.
The pipettes were pulled from the capillary glass using a horizontal pipette puller and the resistance was around 75 M. It was filled with 10 mM ATP disodium in extracellular saline and 1 mM. The AlexFluor will be used for visualization. The solution was kept on ice for an experiment at 20 C, which was stored in aliquots. The tip of the iontophoresis pipette was positioned to be approximately in the medial region of the protocerebral bridge every trial. We recorded from a PFL2 neuron in the trials. We recorded from the same location as PFL2 as part of control trials. The pulse ofATP were delivered using a dual current generator iontophoresis system. The current of 200 nA was used for ejection and the holding current was 10 nA. Visual confirmation of ATP ejection following current pulses was obtained before and after each trial. For the duration of the 10 min trial period, flies viewed a visual cue that moved in closed loop with their rotational movements, as described above. Throughout the trial, pulses were delivered every 30 s with lengths of 100, 200, 300 and 500 ms, repeating in that order.
The circular panorama was built using modular square (8 8 pixel) led panels46. The arena had twelve panels in circumference and two panels tall. The upper panel was removed to accommodate the camera view and light source. The emission of the blue LEDs was chosen to reduce overlap with the GCaMP emission spectrum. Four layers of gel filters were added in front of the arena to further reduce overlap. Two layers of gel filters were used for electrophysiology experiments. On top of the gel filters in both cases we added a final diffuser layer to prevent reflections (SXF-0600, Snow White Light Diffuser, Decorative Films). The visual cue was a bright (positive contrast) 2-pixel-wide (7.5°) vertical bar. The bar had two panels which were full height and half height behind the fly with a single display panel. The bar intensity was set at a 4 with a 0 background luminance.
Experiments used an air-cushioned spherical treadmill and machine-vision system to track the intended movement of the animal. The treadmill consisted of a 9-mm-diameter ball machined from foam (FR-4615, General Plastics), sitting in a custom-designed concave hemispherical holder three-dimensionally printed from clear acrylic (Autotiv). The ball was inflated with medical-grade breathing air through a hole in the holder and used as a flow meter. For machine-vision tracking, the ball was painted with a high-contrast black pattern using a black acrylic pen and illuminated with an IR LED (880 nm for two-photon experiments; M880L3, Thorlabs, or 780 nm for electrophysiology experiments; M780L3, Thorlabs). A ball movement was captured online using either the CM3-U3-13Y3M-CS or CM3-U3-13Y3C-C-CS for two- photon imager or the macro zoom lens InfiniStix. The camera faced the ball from behind the fly (at 180°). Machine vision software (FicTrac v.2.1) was used to track the position of the ball43 in real time. We used a custom Python script to output the forward axis ball displacement, yaw axis ball displacement, forward ball displacement and gain-modified yaw ball displacement to an analogue output device (Phidget Analog 4-Output 1002_0B) and recorded these signals along with other experimental timeseries data on a data acquisition card (NiDAQ PCIe-6363) card at 20 kHz. The yaw ball displacement voltages signal was used to update the azimuthal position of the visual panorama.
ScanImage2021: A Two-Photon Piezoelectric Objective Scanning Scan Head for the Protocerebral Bridge, Fan-Shaped Body, and LAL
Patch pipettes were pulled from filamented borosilicate capillary glass (outer diameter: 1.5 mm, inner diameter 0.86 mm; BF150-86-7.5HP, Sutter Instrument Company), using a horizontal pipette puller (P-97, Sutter Instrument Company) to a resistance range of 9–13 MΩ. 140 mM KOH, 140 mM aspartic acid, 1 mM KCl, 10 mM HEPES, 1 mMEGTA, 4 mM M MgATP were contained in the pipettes. A 0.22 m PVDF filter is used to process Na3GTP and 15 mM neurobiotin citrate.
We used a two-photon microscope equipped with a galvo-galvo-resonant scanhead (Thorlabs Bergamo II GGR) and ×25, 1.10 numerical aperture (NA) objective (Nikon CFI APO LWD; Thorlabs, WDN25X-APO-MP). The goal was to image the head using a fast piezoelectric objective scanner. To excite GCaMP we used a wavelength-tunable femtosecond laser with dispersion compensation (Mai Tai DeepSee, Spectra Physics) set to 920 nm. GCaMP fluorescence signals were collected using GaAsP PMTs (PMT2100, Thorlabs) through a 405–488 nm band-pass filter (Thorlabs). ScanImage 2021. Premium with vDAQ hardware and custom MATLAB scripts were used to control all image acquisition and microscope control. The region for the images of the fan-shaped body and LAL was 150 400. We were able to achieve a volumetric scanning rate of 6–8hertz due to the fact that we obtained 12 slices in the z axis for each volume. For experiments using the selective PFL2 split-Gal4 line, we imaged in the protocerebral bridge, fan-shaped body, or LAL for different trials. For experiments imaging the mixed PFL2 and PFL3 split-Gal4 line, we only imaged in the LAL.
To generate split-Gal4 lines targeting FC2 and PFL3 neurons, we used the Fiji plugin Color MIP tool52 and NeuronBridge53 to find suitable pairs of hemi-driver lines. The cells of interest were generated by the split-Gal4 lines.
Observation of FC2 Neurons by Menotaxis in a CsChromson Meson-Flavored Female Flies in the Bloomington Stock Center
The stock was published by the Bloomington Drosophila Stock Center.
The flies were raised at 25 C. All physiological and behavioural experiments were performed on 1- to 4-day-old female flies. For optogenetic experiments, experimental and control crosses were kept in a box with a blue gel filter (Tokyo Blue, Rosco) as a cover—to minimize exposure to light within the excitation spectrum of CsChrimson while also not keeping the flies in complete darkness; eclosed flies from such experiments were placed onto food containing 400 µM all-trans retinal for at least one day.
To image FC2 neurons during menotaxis experiments (Fig. 1 and Extended Data Fig. 3), we used either +; VT065306-AD/+; VT029306-DBD/UAS-GCaMP7f or +; VT065306-AD/UAS-tdTomato; VT029306-DBD/UAS-sytGCaMP7f.
The expression pattern of VT06912-AD; VT02930-DBD was characterized. 1a,b and 57 C10-AD are included in theExtended Data Fig. 1d,e), VT00355-AD; VT037220-DBD (Extended Data Fig. 1f,g) and 27E08-AD; VT037220-DBD (Extended Data Fig. We crossed each of the lines to UAS-RedStinger.
Source: Converting an allocentric goal into an egocentric steering signal
Welch t-test of flies expressing cs chrimson in PFL3 neurons using blocking and de-gassed brains
We performed a two-sided t-test to assess the change in ipsilateral turning velocity for flies expressing cs chrimson in PFL3 neurons if they only expressed jGCaMP7f. To compare flies expressing CsChrimson in PFL1 neurons with control flies we used a two-sided Welch’s t-test (P = 0.76).
We dissected the brains and incubated them in either 2% paraformaldehyde (PFA) for 55 min at room temperature or in 1% PFA overnight at 4 °C. We blocked and de-gassed brains in a blocking solution consisting of 5% normal goat serum (NGS) in 0.5% Triton X-100, phosphate buffered saline (PBT).
For the GFP and RedStinger labelling experiments. 1a,b,d–i), we used a primary antibody solution of 1:100 chicken anti-GFP (Rockland, 600-901-215), 1:500 rabbit anti-dsRed (Takara 632496) and 1:10 mouse anti-Bruchpilot (nc82, DSHB) in 5% NGS/PBT and a secondary antibody solution consisting of 1:800 goat anti-chicken:Alexa Fluor 488 (Invitrogen A11039), 1:400 goat anti-rabbit: Alexa Fluor 594 (Invitrogen A11037) and 1:400 goat anti-mouse:Alexa Fluor 633 (Invitrogen A21052) in 5% NGS/PBT. For the data in theExtended Data fig. We used a primary and secondary solution of rabbit and goat anti-TNTs.
For visualizing biocytin-labelled neurons after patch-clamp experiments (Extended Data Fig. 6a), the primary antibody solution we used was 1:10 mouse anti-nc82 in 1% NGS/PBT and the secondary antibody solution was 1:800 goat anti-mouse:Alexa Fluor 488 and 1:1,000 streptavidin:Alexa Fluor 568 (Invitrogen S11226) in 5% NGS/PBT.
Brains were mounted in Vectashield and images were acquired using a Zeiss LSM780 confocal microscope with a 40×/1.20 NA water-immersion objective or a 10× air objective.
Source: Converting an allocentric goal into an egocentric steering signal
Closed-loop wind experiments with a rotating spigot and a manifold around a fly in the vicinity of an LED arena
For closed-loop wind experiments we used a virtual reality setup with a device that delivered wind from 36 directions around the axis, first described in ref. 45. The design of this device took inspiration from past wind-delivery devices for Drosophila56,57,58,59. In brief, the wind device consisted of two separate parts: a circular manifold surrounding the fly and a rotating spigot, which could deliver wind to the tubes in the manifold. Outside the arena was placed a rotating spigot. The components were made from 3D printed parts. The circular manifold had 36 equally spaced openings and these were connected to the rotating spigot via 36 transparent plastic tubes (internal diameter 1/16 inch, Tygon E-3603, Saint-Gobain). The spigot received pressurized, filtered, air from the wall, whose flow rate was regulated by a mass flow controller (Alicat Scientific). The air in the manifolds is expelled by tubes in the spigot and a motor is used to change that. Because the spigot’s nozzle was 20° wide, it spanned two to three openings at any one time. The position of the spigot was controlled in closed loop with the yaw rotations of the ball using the same controller system used to update the position of the vertical blue bar on the LED arena. The wind rotating around the fly did not present a visual cue because the tubes were fixed. The flow controller was used to turn the air on and off over the course of an experiment. There were no wind control experiments during the ‘wind period’, in which the air was set to 1 standard litres per minute. The data collected on two separate rigs was constructed to be as close to perfect as possible.
Wild-type flies are the most likely to menotaxis when they are deprived of food for 8–16 h and heated to 34 C. The study showed that if the same level of food deprivation is maintained, some people will produce flies that are bad. We went for a shorter period of food deprivation. After tethering flies, we usually do at least 3 h of experiments. During this interval, we kept tethered flies inside a box with a wet piece of tissue paper to prevent desiccation. For FC2 stimulation experiments, we placed flies on plain agarose roughly 14 h before tethering. In all plate-tethered experiments, we heated the tethered fly using a closed-loop temperature control system. For pin-tethered experiments, we heated flies using a 980 nm infrared diode laser (RLDH980-200-3, Roithner). The intensity of the laser was controlled via pulse-width modulation in closed loop with a temperature reading from a thermal camera image (C2, Teledyne FLIR). The temperature set points were assigned for the two experiments, and both were 35 C.
Source: Converting an allocentric goal into an egocentric steering signal
Wind Directions for allocentric air-delivery spigots from extended data to the detection of changes in the FC2 phase position
The mean of the differences between the bar position and the spigot angle was used to calculate the wind direction for each trial. This value was not necessarily identical to the nominal allocentric wind direction set by our code because of inertial/mechanical latencies associated with the air-delivery spigot needing to physically rotate to deliver air from a new direction. The set point and trial-computed allocentric wind direction could differ by up to 13°.
The data was fit to the A, H, and V parameters in the extended data figs.
For extended data. If the stimulationROI had at least one chip within the boundaries of the stimulationROI Scan path and was otherwise considered, it was a stimulationROI. In extended data 4d, we only analysed ROIs that were outside the stimulation ROI. The mean F/F0 and the mean F /F0 were divided during the 30th and 5th stimulation periods to arrive at the change in the column. To calculate anROI’s distance from the stimulation site, we first defined the stimulation site as the column ROI with the highest fraction of pixels inside it. For each ROI we then computed its wrapped distance in number of ROIs. For instance, column ROI 2 and column ROI 15 have a (wrapped) distance of three, given that there are 16 columns in our analysis. Our stimulationROI could overlap with other ROIs, in extended data fig. 4d, there are no column ROIs with a distance of one.
In Extended Data Fig. 3f,g, rapid changes in the FC2 phase position were detected by finding peaks in the filtered phase velocity (500-ms boxcar filter) using the SciPy65 function signal.find_peaks. The FC2 PVA within 1 s from the peak phase velocity had to be above 0.15 at all time points and the mean PVA had to be over 0.25. Criteria helped make sure that genuine changes in the FC2 bump position were detected rather than spurious changes in the FC2 phase because of a poorly estimated phase. To overlay all of the detected changes in FC2 phase position, as well as the flies’ heading during these moments (Extended Data Fig. We were able to align traces to the start of the peak. We inverted the FC2 phase for traces where the peak phase velocity was positive, in order to combine them.
The FC2 phase in the fan-shaped body was computed by using the average of the population coefficients. We computed the EPG phase in the protocerebral bridge as described previously4,34. For each timepoint, we treated the glomeruli ΔF/F0 in the bridge as a vector of length 16 and took the Fourier transform of this vector. The phase of the Fourier spectrum at a period of 8.5 glomeruli was used as the EPG phase.
To correct for motion artefacts, we registered two-photon imaging frames using the CaImAn64 Python package. We used Python to write a graphical user interface for the LAL’s left and right sides. The local correlation image of each Z slice was used to draw the ROIs. In the case of the fan-shaped body, we used a semi-automated method to define columns as described previously4. In brief, we first defined an ROI including the entire fan-shaped body. This ROI was then subdivided into 16 columns of equal angular size using two lines that defined the lateral edges of the fan-shaped body. For each ROI, we defined ΔF/F0 as equal to (F − F0)/F0, where F is the mean pixel value of an ROI at a single timeframe and F0 is the mean of the lowest 5% of F values.
The yaw, pitch, and roll angles of the ball were sampled at 50 Hz, and aligned to our imaging data files using the ball camera’s trigger signal. We shifted the acquired ball-position data backward in time by 30 ms due to our measured latency between the trigger pulse for acquiring a frame and when FicTrac finished processing the image. For behaviour only closed-loop wind experiments—which did not require aligning behavioural and neuronal data—no camera triggers were used and all signals were downsampled to 50 Hz.
All time series data were digitized with a Digidata 1440 A (Molecular Devices) at 10 kHz using the PClamp software suite (Clampex 11.1.0.23 and Axoscope 10.7.03), except two-photon images, which were saved as tiff files using ScanImage at frequencies ranging from ~4-10 Hz, as described above. The end of a frame is marked by the y galvo flyback which is an alignment point. For each imaging volume, the midpoint between the start of the volume’s first z-slice and the end of its last z-slice was used as its time stamp.
For Fig. 2, we alternated between stimulating one of two positions in the fan-shaped body (referred to as location A and B). When we didn’t wish to cause fan shaped areas in the body, we moved the stimulationROI to a more anterior position in the brain with no Cs chrimson-tdTomato expression. 4b). This approach ensured that the average laser power per volume remained constant throughout the experiment, which is important because flies could show behavioural reactions to changes in illumination intensity. In the experiments, we used a stimulation power of 50 mW. The stimulation return was present in all three slices. The acquisition rate was 3.32 Hz. The duty cycle was ~0.67 (the number of pixels in the stimulation ROI divided by the total number of scanned pixels). The locations of the stimulators and the brain scans were adjusted between the recordings if we picked up more than one fly.
Source: Converting an allocentric goal into an egocentric steering signal
The FC2 and EPG phases in flies and their differences after laser irradiation with a 16/0.8 NA gaAs/c mirror
The FC2 phase changed relative to its position immediately prior to the bar jump, we performed a Rayleigh test for uniformity, with a means angle of zero. To assess whether the EPG phase tracks the bar during a bar jump we performed a V-test with μ = 90° (P = 7.99 × 10−3). The same tests applied to Extended Data Fig. 3e yielded μ = 0° (P = 7.69 × 10−8) for the FC2 phase and μ = 90° (P = 2.49 × 10−5) for the EPG phase.
In Fig. 6f and Extended Data Fig. 11b,c, the absolute distance to wind was taken to be absolute value of the flies’ heading relative to the wind direction, computed as described above.
There are some changes indicated below from the patch-clamp experiments we performed. We perfused the brain with an extracellular solution61 bubbled with carbogen (95% O2, 5% CO2). The amount of the solution in mM was as follows: 103 NaCl, 3 KCl, 5 TES and 10 trehalose dihydrate. The compound in the solution was 140 potassium aspartate, 1 KCl, 10 HEPES, 1 EGTA, 0.5 Na3gTP, 4 MgATP. For some recordings the solution also included 13 mM biocytin hydrazide (Invitrogen, B1603) and 20 mM When we were recording the identity of the cell, we used an animal tranquilizer that could be filled with a molecule called Invitrogen.
Changes are indicated below after we performed two-photon calcium irradiation. The laser we used was called the Clarifyer, which is specific to the minimum wavelength of 928 nm. We used a galvo-Galvo mode to move a 16/0.8 NA objective along thez axis and did it with Cambridge Technologies MicroMax. Emission light was split using a 565 nm dichroic mirror. We used a 500-550 nm bandpass filter for the green signal and a 590–650 nm bandpass filter for the red signal. Emission photons were detected and amplified using GaAsP detectors (Hamamatsu, H10770PA-40). ScanImage60 (2018b) software was used to control the microscope.
For Fig. 5a–d, we used ScanImage’s MultipleROI feature to define two 50 × 50-pixel ROIs for each side of the LAL. We used two slices of the LAL to produce a 9.16 Hz volume rate. For Fig. 1, we scanned the protocerebral bridge or the fan-shaped body at 4.95 Hz using a 128 × 64-pixel ROI with 3 z slices. We used the laser power of 25 mW in the standard experiment. Imaging recordings lasted up to 26 min. Occasionally, the fly’s brain would slowly sink over the course of a recording. To correct for this motion, we manually adjusted the position of the objective via a microscope-stage motor during the recording.
We alternated between stimulating the left or right LAL. Between trials, we moved the stimulation ROI to the LAL that did not have cs chrimson-tdTomato. The experiments used stimulation power of 70 mW. We used a single z-slice to scan the LAL with an acquisition rate of 4.97 Hz and the duty cycle was ~0.33.