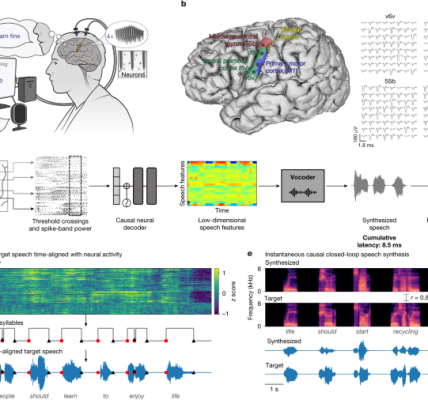
Do not take Cobenfy as a placebo, but it might help. Tiffany and Andrew Miller, a psychiatrist in schizophrenia, explains a possible alternative therapy
She says to just keep trying. It’s hard to go on and off medication, but when you find the right one, it makes a huge difference.
She says she doesn’t like the fact that when she has an episode, she loses some bits of herself and her husband. “So if I could have something that would help me have a little bit more initiative, that would be wonderful.”
Tiffany is interested in trying the drug, down the road. Previous drugs only tackled the so-called positive symptoms of schizophrenia, like delusions and hallucinations, such that Cobenfy has been shown to decrease the negative symptoms.
The FDA based its approval on 5-week double-blind, placebo-controlled studies. That means some patients received Cobenfy and others got a placebo, but neither the patients nor the clinicians knew which was which until the study was over. The short study duration prompted some to point out questions about the long- term safety and efficacy of the drug.
He has patients and their parents excited about a possible new treatment option, he says. Ballon is working on an ongoing study of how Cobenfy fits in with existing drugs and whether they can be used together.
Andrew Miller’s strategy to revive the therapy was devised years later. He recognized that administering the muscarinic-activating agent together with another compound that blocks xanomeline’s effects outside the brain could maintain the cognitive and antipsychotic benefits without causing severe gastrointestinal distress.
Miller’s team decided to add a second medicine, already used for overactive bladder, in order to shut down the gastrointestinal receptor. The trick: That medication can’t cross into the brain from the blood.
The Story of Cobenfy, a Psychotherapist Who Suggests Dopamine as a Medicine for Schizophrenia
She’s talking about Miller’s company, Karuna Therapeutics, which was acquired by pharmaceutical heavyweight Bristol Myers Squibb for $14 billion dollars earlier this year.
Bristol Myers Squibb says the drug will be available starting in October at $1,850 a month, which is in line with other schizophrenia treatments. It’s unclear how easy it will be for patients to get insurance coverage for Cobenfy.
If it is like a lot of other new medications, insurance may mandate that people try at least two generic medicines before they pay for it, according to Dr Jacob Ballon.
The neurotransmitter dopamine is associated with reward and learning, but there are other functions to it. It also plays a role in controlling movement, for example — that’s why that one drug made Tiffany pace.
Shinn says the dopamine hypothesis suggested thatSchizophrenia is associated with too much dopamine activity.
The first drug used to treat psychosis act on the same chemical that helps the brain communicate with the rest of the body: dopamine.
Then, she played what she calls the “meds game,” trying different pills until one worked for her. But some of the side effects were brutal. Common antipsychotic drugs can cause weight gain and increase the risk for diabetes.
KarXT: A First-Name Solution to Schizophrenic Effects in a Psychopathist’s Dilemma
“I was opening presents — everyone was happy. And I’m just sitting there like, there’s nothing going on. Like, I’m staring at a blank wall,” she says. I told everyone that I was better.
She was put on a drug that made her feel like a zombie. She was not familiar with herself while watching a video of her birthday party.
That difference has captured the attention of patients like Tiffany, a librarian in Oklahoma. She asked us to use only her first name because of the stigma associated with schizophrenia.
The once-a-day pill will be called Cobenfy and has previously been referred to as KarXT. It appears to have fewer side effects than current medicines.
In clinical trials, the two-in-one pill outperformed a placebo in relieving characteristic symptoms of schizophrenia3,4, without the weight gain, sedation or movement issues that are commonly associated with existing antipsychotics. The side effects of KarXT were largely limited to gut disturbances, which tended to resolve after a week or two of daily use.
“This is a revolution of the treatment of psychosis and I’m not saying it’s lightly.” says Christoph Correll, a psychiatrist at the Zucker School of Medicine in New York. People who haven’t been helped with traditional drugs will now be able to be treated. That is very exciting.
Ann Shinn, a psychiatrist at a hospital who has no commercial ties to KarXT, says that this is a completely different option than the ones they currently have.
Karuna Therapeutics: a long-term therapy for schizophrenia using xanomeline, trospium, and other long-acting drugs
Trials showed that the drug offered both antipsychotic and cognitive benefits1,2. But xanomeline also caused nausea, vomiting and stomach pain — because muscarinic receptors are active in the gut as well as the brain — leading Lilly to ultimately shelve the drug.
In 2009, Miller formed a company called Karuna Therapeutics, based in Boston, Massachusetts. Karuna used xanomeline and trospium. The molecule doesn’t interfere with xanomeline’s action in the brain, and it blocks muscarinic receptors, meaning that it prevents side effects in the gut.
The drug has a few shortcomings. It requires twice-Daily administration, and studies show there is a link between frequent dosages and higher levels of non-adherence and treatment discontinuation in people with schizophrenia. It is difficult to find a long-acting injectable antipsychotic that requires only a few doses annually.
Concerns are being raised about KarXT’s cost-effectiveness compared to alternatives, with an expected price tag of $20,000 per year. Despite this, most industry analysts predict strong demand, with peak annual sales projected in the billions. This potential drove Bristol Myers Squibb (BMS) in Princeton, New Jersey, to acquire Karuna for approximately $14 billion this year.
Steven Paul is a psychiatrist at Washington University School of Medicine and he is excited about discovering the best ways to harness this therapeutic strategy.
“Now we have new biology and new pharmacology to explore,” he says. “It will be fun and scientifically relevant — and, hopefully, clinically beneficial to patients — to find out.”
The medication is the first in decades to have a different mode of action than do current drugs, achieving better symptom relief with fewer side effects.