Nodes with More than 15 Identical Sequences are Responsible for Variable Mutation Rates in GC B Cells Induced by Modified Kinase 2 Activity
Among the expanded clones, the fraction of nodes containing either 1, 2–15 or more than 15 genetically identical sequences were 50%, 47% and 3%, respectively (Fig. 2c and Supplementary Table 2). Thus, only a small fraction of all nodes have more than 15 identical sequences. 18% of GC B cells are found in cells with at least fifteen identical sequences if they are considered independently. The GC reaction can be contributed by a large assortment of identical progeny.
The activity of the cytidine deaminase causes SHM. AID has a poor catalytic activity and even small changes to AID expression can change the SHM36. To determine whether variable mutation rates in GC B cells were mediated by altered AID expression in expanded nodes, we profiled its expression in NP-OVA and RBD-elicited GC B cells contributing to nodes of sizes 1, 2–15 or more than 15 (Extended Data Fig. 8a–c). Despite the absence of SHM in grossly expanded nodes, there were no significant decrease in Aicda transcript levels in the larger node classes. Thus, differential Aicda expression levels are not likely to be responsible for the absence of mutation in large nodes.
The G0 and G1 phases of the cell cycle are characterized by low activity of cyclin-dependent kinase 2 (CDK2), which, when partnered with cyclin E, promotes progression through G1 and S-phase initiation43. To measure these phases in a way that mimics them in humans, we used mice carrying a real-time reporter of CDK2 activity. 3b,c). This reporter, based on previously published constructs43,44, consists of amino acids 994–1,087 of human DNA helicase B (DHB) fused N-terminally to the tdTomato red fluorescent protein and inserted into the ubiquitously expressed Rosa26 locus (Rosa26DHB-tdTomato; Fig. 3c). The DHB domain translocates from nucleus to cytoplasm after phosphorylation by CDK2. It therefore localizes to the nucleus when CDK2 activity is absent during G0, gradually exiting to the cytoplasm as cells progress through G1, S and G2 phases43,45 (Fig. 3b,d). The cytoplasmic-to-nuclear (C/N) ratio of DHB–tdTomato fluorescence is used to determine the CDK2 activity. The B8hi B cell Receptor and histone 2B were crossed to the mice to visualize their activity.
We gave H2b-mCherry mice with 4-Hydroxy 3-nitrophenylacetyl and administered DOX and mCherry indicator dilution on the day 12.5 to track GC B cell division. 2a,b). GC B cells that had divided on an average one or fewer times were the majority at the time of DOX administration. 1c and 2b).
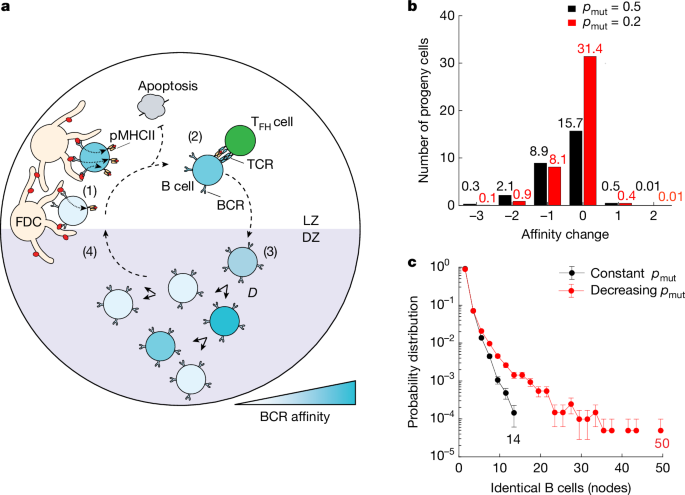
The contribution of clonal bursts to the growth rates of branched tree biomarkers in the constant pmut model
Our modelling predicts that a constant mutation rate of around 1 × 10−3 per base pair per cell division1, equivalent to approximately 50% chance of mutation per division pmut = 0.5, should produce branched trees containing limited small collections of identical sequences (nodes) of 15 or fewer cells (Fig. 1c, black). The decreasing pmut model predicted trees with up to 50 cells, had the same simulation parameters. 1c, red). There were trees with 2–15 members as well as trees with much larger ones that had 15–15 identical members. 2f. The changes in the pmut model could not be accounted for by the vastly expanded nodes, which were more in line with the model.
The use of profiling the mCherryhigh and mCherrylow B cells is useful in comparing the number of times they have divided versus the amount of DOX exposure. However, as many GC cells were mCherryintermediate, many GC B cells were excluded from this analysis. We performed a single cell analysis of GC B cells to understand the contribution of clonal bursts to total GC responses. 6a–c. The large nodes containing identical sequences that contributed to affinity maturation were observed, consistent with the data obtained by mCherry fractionation. 6d–g). As expected, the grossly expanded nodes of B cells with more than 15 identical sequences also contributed disproportionately to the overall number of B cells and were highly enriched within high-affinity (RBD+) clones (Extended Data Fig. 6e–g). Heterogeneous expansion contributes to affinity maturation in GC reactions.
Finally, we considered whether stochasticity in the constant pmut model could account for the large nodes observed in experimental data. We used a simulation of the case with the stochastic pmut. 7a), and the case where the number of divisions corresponding to a constant level of T cell help was stochastic (Extended Data Fig. 7b). We found that neither scenario could account for the long-tail behaviour of node sizes observed in experiments. Experiments and theory show that the per division rate of SHM is regulated and decreases with increasing T cell help.
AID introduces a C>U mutation at a preferential sequence of nucleotides that make it more likely it will be used. To determine whether absence of SHM in expanded nodes was due to previous loss of these motifs, we profiled AID hotspots between cells contributing to nodes of sizes 1, 2–15 or more than 15. The motifs were intact in all of the cells in the class. 8d–f). Differences in SHM between the two locations do not owe their existence to target motif decay. Finally, we compared the level of cell death among mCherry compartments and found no significant differences (Extended Data Fig. 8g,h).
The idea that strong selection signals reduce relative time DZ cells spend in G0/G1 and reduce their exposure to AID is consistent with these data.