Experimental Investigation of Human Coronaviruses Associated with Syrian Goldenhamsters (zoonotic SARS CoV), WIV-1 and SHC014
The antiviral assays against Pg-CoV, WIV-1 and SHC014 (zoonotic sarbecoviruses) were conducted in A549-hACE2 cells, as previously described61,62,66,67. Antiviral assays against other human coronaviruses are detailed in the Supplementary Methods.
The housing conditions and experimental procedures were approved by the ethics committee of Johnson & Johnson Research & Development. 5 animals per group was needed to obtain statistically significant significance in the Syrian golden hamster studies. After arrival, the animals were randomly assigned to groups. No blinding was performed during the experiment. Female Syrian golden hamsters (Janvier Laboratories) aged 8–10 weeks were anaesthetized by isoflurane inhalation and inoculated intranasally with 100 μl of PBS containing 1 × 104 TCID50 SARS-CoV-2 (day 0). The animals were treated in three different ways. and continued to be dosed BID at 10 h intervals with vehicle or JNJ-9676 (75 mg per kg per dose in PEG400) (Fig. 3f). The animals were dosed BID at 08:00 and 16:00. On day 4 after infection, the hamsters were euthanized by CO2 inhalation. The lungs were homogenized using a tool called the Precellys homogenizer. Viral RNA and infectious virus levels were quantified in the lung homogenate supernatant by RT–qPCR and end-point virus titration, respectively (Fig. 3g,h). RNA was extracted using the MagNA Pure 96 DNA and Viral NA Small Volume Kit following the Viral NA universal SV 4.0 protocol (Roche). The LightCycler Multiplex RNA Viruses Master kit was used as a primer and as a probe for the experiment. The lung homogenate was prepared for end-point titrations in 1 MEM with 2% FCS, 2 mM alanyl-glutamine and 0.04%. This dilution series was then added to confluent Vero E6 cells in a 96-well plate and incubated for 72 h at 37 °C. The infectious viral titres of the samples were determined by microscopically scoring the virus-induced cytopathic effects and quantified as the TCID50 ml−1 according to the Reed–Muench calculation method73. The TCID50 ml−1 values were normalized to the total weight of the right lung and expressed as TCID50 per mg tissue.
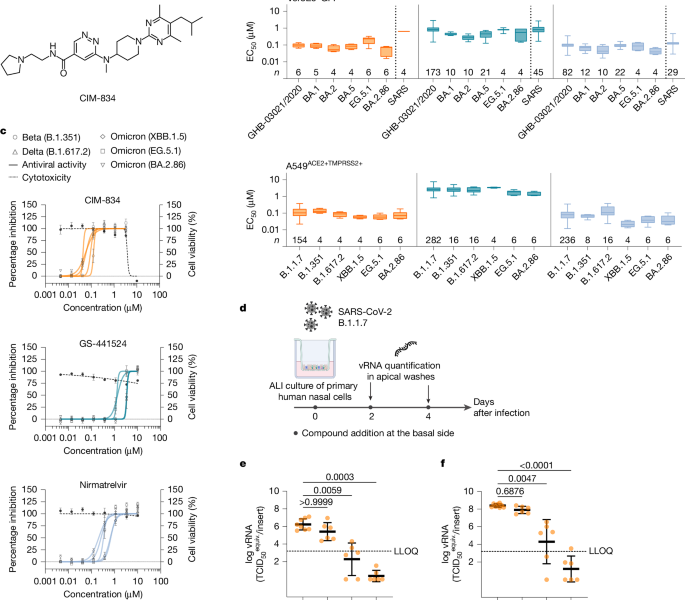
A representative image of nirmatrelvir synthesis: In vivo and in vitro studies and the effects of CIM- in human airway cells
In Extended Data Fig. 2g, uncut western blots are shown of purified M proteins. There was a time when these were created as a quality control.
In Extended Data Fig. There is a micrograph from the data collection. To obtain this representative image, 12,988 images were taken.
The synthesis of JNJ-9676 is described in patent WO-2024/008909 and in the Supplementary Methods. nirmatrelvir was synthesized according to literature procedures at the MedChemExpress. For in vitro experiments, JNJ-9676, molnupiravir or nirmatrelvir was dissolved in 100% dimethyl sulfoxide (DMSO) as a 5–100 mM stock. For in vivo experiments, JNJ-9676 was dissolved in 100% polyethylene glycol 400 (PEG400) as stocks of 75, 25 or 8.33 mg ml−1, molnupiravir was dissolved in 100% PEG as a stock of 300 mg ml−1 and nirmatrelvir as a stock of 250 mg ml−1.
Procedures were followed. Human airway cells were obtained from Epithelix in an air–liquid set-up. After arrival, the inserts were washed with pre-warmed 1× PBS and maintained in MucilAir medium (Epithelix, EP04MM) at 37 °C and 5% CO2 for at least four days before use. The cultures were treated with a mixture of different concentrations for 1 h before infections with 100 l (1,000 TCID50 per insert) at the apical side. The viral releases from the cultures were measured by washing the apical sides with 250 l Mucilair Medium and by using a test to determine the load. Compound-containing medium in the basolateral side of the cultures was refreshed on day 2 after infection. All incubations from start of the infection were done at 35 °C and 5% CO2.
InvivoGen cells were passed in the presence of increasing concentrations of a particular substance called CIM- . The virus was cultured at the same concentration and at higher concentrations after it was picked up. The culture with the highest compound concentration that still showed the breakthrough was used for the next passage. At passage 5 (day 17), vRNA in the cell-culture medium was sequenced.
Recombinant viruses, derived from infectious clones of PgCoV GD/2019, RsSHC014, and WIV-1 expressing nanoluciferase, were derived and isolated as working stocks as previously described60,61,62.
Toxicity on day 5 in uninfected VeroE6-mCherry cells injected with SARS-CoV-2 GHB
Cytotoxicity was evaluated on day 5 in treated but uninfected cells using an MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) reduction assay65.
Toxicity was assessed using anEVOM3 to determine if there was a risk of toxicity from exposing noninfecting inserts to the same JNJ-9676 concentration as for antiviral treatment. Brefeldin (0.3 µM; internally synthesized) was used as a toxicity control.
VeroE6 cells were seeded in 12-well plates the day before infection. Cells were infected with an MOI of 1 TCID50 per cell (SARS-CoV-2 GHB) and compounds were added 0, 3, 5 and 7 h after infection. At 10 h after infection, virus-containing supernatant was removed and cells were either lysed for quantification of vRNA levels by RT–qPCR (see above) or cells were collected for determination of intracellular infectious virus titres by plaque assay (see below). For the time-of-drug-addition assay with reporter cells and reporter virus, VeroE6–mCherry cells were seeded in 96-well plates the day before infection. After cells were bitten by the mRhinoGreen virus, compounds were added to the cells for a duration of 8 h. At 10 h after infection, cells were imaged using high-content imaging and the number of infected cells was calculated, after which the percentage inhibition relative to the DMSO-treated control was calculated.
FabB2 truncated C terminal with a linker and its use as a block to block dimer formation in low-protein filter plates for offline ASMS
A truncated C terminal with a linker was added to the heavy chain of FabB2 in order to block Fab dimer formation. The sequence encoding the light chain of FabB2 was cloned into a pcDNA3.4 vector with an added N-terminal gLUC signal sequence (MGVKVLFALICIAVAEA).
Conditioned medium was loaded onto a 10 ml HisTrap excel column is flowing at a rate of 8 million liters per minute. The column was washed with a buffer of 20 mM. NaCl, 20 mM imidazole) and eluted with over 5 CV using a 39.2–500 mM imidazole gradient prepared in buffer (20 mM sodium phosphate pH 6.5, 150 mM NaCl, 500 mM imidazole). Peak fractions of FabB were subsequently purified on to a HiLoad 16/600 Superdex 75 pg column (Cytvia) in buffer (20 mM sodium phosphate pH 6.5, 150 mM NaCl).
Experiments were performed in a total volume of 10 µl. The instrument was used to measure the melting temperatures. The samples were prepared in a well plate with JNJ-9676 and 50 M of coliform in 20 mM HEPES. NaCl is 0.061%, 0.0001%, 00.000, 0.00033%, and 1% DMSO. The samples loaded into standard‐grade glass capillaries were measured under a temperature range of 25–95 °C with a temperature gradient of 1 °C min−1, and the intrinsic protein fluorescence at 330 and 350 nm was recorded. The data was analysed using PR.ThermControl v.2.1.6.
The sample types for the ASMS experiment were compound QC, the M Protein, and no-protein control.
For the preparation of SEC filter plates for offline ASMS, 130 µl of pre swollen Bio-Gel P10 resin slurry was added to each well of a low-protein-binding Millipore HTS 384 HV filter plate (hereafter, size-exclusion plate) with a 0.45 µm Durapore (PVDF) membrane (MZHCN0W10). The flowthrough was discarded after the size exclusion plate was placed into the 4 C refrigeratedcentrifugation for 2 min at 1000g. Each item was washed four times on top of a buffer containing 20 mM HEPES. Flowthrough from the washes was discarded after beingcentrifugation at 1,000g for 2 min. The ASMS assay plate was prepared using an Echo acoustic liquid handler, and an aliquot of 20 nl of 5 mM compound dissolved in 100% DMSO was transferred from the source plate into four separate wells of a 384-well, natural, polypropylene V-bottom plate (781280). An aliquot of purified recombinant M protein stock solution was thawed on ice, then diluted using assay buffer to a working concentration of 5 µM and 2% DMSO. Then, 20 µl of the resulting working protein stock was dispensed into three wells containing compound to yield a final concentration of 5 µM (3 technical replicates). To control for compound breakthrough of the SEC resin, either in-solution or through micelle partitioning, a separate working stock was prepared without protein and dispensed as a 20 µl aliquot into the remaining compound well. The plate was centrifuged at 1,000g for 1 min at room temperature and incubated at 25 °C for 30 min.
All of the samples were transferred to the size-exclusion plate, which was quickly centrifuged at 1,000g for 2 min at 4 °C to minimize compound breakthrough. The resulting flowthrough was diluted with 15 µl MS-grade water (Honeywell) to reduce the detergent concentration and centrifuged further at 2,000g for 5 min at room temperature to collect any insoluble precipitate.
The 5 mM compound was transferred from the source plate to the wellplate with 25% acetonitrile and 2% DMSO in the solution.
We put 5 M M in buffer only for 30 minutes at 25 C. NaCl, 0.001% LMNG, 0.0001% CHS, 0.00033% GDN, supplemented with 2% DMSO) or in buffer with CIM-834 (in DMSO, 2% final concentration). The compound was removed from the bound compound by the Zeba Spin Desalting columns. All experiments were done in triplicate. Mass-spectrometry measurements were done on an Agilent 6546 Quadrupole Time-of-Flight for liquid chromatography with tandem mass spectrometry. For data analysis, the Find by Formula method for compound identification was used. An EIC peak area of [M + H]+, [M+Na]+ and [M + K]+ masses was extracted using a mass error tolerance window of 10 ppm.
The data was processed using a mass error tolerance window of 3 ppm.
Cryo-EM Molecular Dynamics Modification of Cosmic Structures (PDB: 8CTK, Alphafold2 and B) for the Design of the M-Fab-B Map
The PELCO easiGlow Discharge Cleaning System is used for the discharge of glow discharge from the mesh grids. A total of 3 l M samples were applied to the EM grids using the following settings. Flash-freezing in liquid ethane cooled by liquid nitrogen was performed. Cryo-EM data collection was automated on the 200 kV Thermo Scientific Glacios microscope controlled by EPU software. Micrographs were taken with a Facon4 detector and in counting mode. Each exposure had a dose of 40 e 2 and recorded 40 frames. The digital micrographs were measured at 0.910.
The image quality was monitored with the use of cryoSPARC Live. Image preprocessing steps, including patch motion correction, patch contrast transfer function (CTF) estimation, blob particle picking (100–200 Å diameter) and extraction, were performed simultaneously. During a four day session, over 12,000 raw micrographs were recorded using the Glacios microscope. There were templates for particle repicking in acceptable 2D classes. A round of live 2D image classification yielded over a million good particle images. 3D reconstruction was used for these particles. The first round of five starting 3D models were calculated, resulting in one major 3D class, followed by a second round of four 3D classes. A class was refined to a 3D em map with an average resolution of 3.06 after being refined using 484,610 particles.
Resolutions were estimated by applying a soft mask around the protein complex density using the gold-standard (two halves of data refined independently) FSC = 0.143 criterion. Before visualization, all density maps were sharpened by applying different negative temperature factors along with the half maps and used for model building. ResMap was used to determine local resolution. Detailed statistics about the cryo-EM data processing can be found in Extended Data Fig. 6a–f.
Initially, a previously determined SARS-CoV-2 M structure (PDB: 8CTK; ref. 34) and an Alphafold2 model62 of Fab–B were rigid-body fitted into the M–Fab–B-CIM map using the UCSF Chimera Fit in map tool63. An initial round of molecular-dynamics flexible fitting was then done on the combined model using Namdinator64 and then manually adjusted in Coot65. During the latter step, the CIM-834 compound was added using the ligand builder tool. When selecting the pose of CIM-834, the following were taken into account. First, the morphology of the density. The flat plate-like morphology of the density orientated away from the M two-fold symmetry axis was consistent with the piperidine and pyrimidine rings of CIM-834. The density for the opposing end of the density was thinner and less well resolved (Extended Data Fig. 6), consistent with the increased flexibility of the molecule beyond the amide position. The positioning of hydrogen-bonding partners is second. The pyridazine ring is placed in proximity to S99 and N117 due to a selected orientation. The model was then refined by iterative cycles of manual model building, using Coot65, real-space refinement, and eLBOW. Model validation was done using a model called Molprobity68.
A statistical analysis of lung histopathology with a multivariate analysis. The case of the Lung Viral-Load Data
The lungs were fixed overnight and used for histological examination. After staining the tissue sections, a pathologist used a microscope to see if there was lung damage. The scored parameters, for which a score of 0–3 was attributed, were the following: congestion, intra-alveolar haemorrhagic, apoptotic bodies in the bronchus wall, necrotizing bronchiolitis, perivascular oedema, bronchopneumonia, perivascular inflammation, peribronchial inflammation and vasculitis. The cumulative score was reported after the different scores had been reported.
The statistical analyses were done using R and GraphPad. A log10 transformation was applied to the lung viral-load data (RNA and infectious virus) to approximate normality. The mean differences between the treatment groups and the vehicle groups were calculated using a one-way analysis of variance and dk’s multiplicity correction.
In the case that normality could not be assumed for the outcome variable or in case of lung histopathology, the nonparametric Kruskal–Wallis test by ranks was applied. The post hoc Dunn’s test with the Benjamini–Hochberg’s multiplicity correction was applied to account for multiple testing. A significance level of 0.05 was used.
All graphs were prepared using Graph Pad spil. Figures and schemes were created using BioRender.com and Adobe Illustrator 28.1 (Windows).
There were various statistical tests used in the study and they are mentioned in the figure legends. The 2D Ems for quantification were obtained from three independent preparations. The HPF samples that were prepared in one preparation were the exception to the rule when it came to Tomography.
The determination of the microsomal stability of the nsp-8L7 compound and of its diluted dilute monomers in a biomek I5 automate
Compound microsomal stability was determined using mouse microsomal fractions (Gibco; final protein concentration, 0.5 mg ml−1), with hamster microsomal fractions (Xenotech; final protein concentration, 0.5 mg ml−1) and with human microsomal fractions (Xenotech; final protein concentration, 0.5 mg ml−1) at a substrate concentration of 1 μM with or without NADPH (final concentration, 1 mM). cubations were done for 120 min in a row. At various time points (5, 15, 30, 60 and 120 min), 25 μl of the reaction mixture was sampled and quenched in 300 μl of acetonitrile containing internal standard. The suspension was whirled, and the supernatant was mixed with water. The resulting solution was analysed by liquid chromatography with tandem mass spectrometry to determine the half-life of the compound.
The IC50 was determined by using a formula which takes 100 as the percentage of active activity in the molecule and I2 as the percentage of activity in the molecule. curve fitting was used to find the IC50. Results were obtained from each measurement.
Adding 20 l EDTA (100 mM) stopped the reaction assays. There were positive and negative controls that used a reaction mix of 5% and 100 mM instead of compounds. The process of transferring reaction mixes was done using a Biomek I5 automate. The manufacturer’s instructions instructed that 60 l of PicoGreen reagent should be distributed into each well of the Greiner plate. The plate was incubated for 5 min in the dark at room temperature and the fluorescence signal was then read at 480 nm (excitation) and 530 nm (emission) using a Tecan Safire2 and/or a ClarioStar.
The reactions were done on a well with a 96-well Nunc plate. All experiments were robotized by using a BioMek I5 automate (Beckman). 2 l of eachDiluted compound was added to the Concentration in wells. After an 8-min incubation at room temperature, the nsp8L7 + nsp8) mix was distributed in wells to form the active complex. Nsp12 was then added to wells and incubated for 8 min. Reactions were started by adding the UTP + poly(A) template mix and were incubated at 30 °C for 20 min, using 350 nM of poly(A) template and 750 µM of UTP final concentration.
The compound concentrations leading to 50% inhibition of polymerase-mediated RNA synthesis was determined in IC50 buffer (50 mM HEPES, pH 8.0, 10 mM KCl, 2 mM MnCl2, 2 mM MgCl2 and 10 mM DTT) containing seven increasing concentrations of compound (from 1 µM to 100 µM) and 150 nM of nsp12 (ref. 76) in complex with 450 nM nsp8 and 450 nM nsp8L7 (ref. 77).
The activity and inhibition were determined using black 384-well HiBase non-binding plates. In a single step, inhibitor concentrations were increased with the addition of a 5 M fluorescent synthetic peptide in the HEPES buffer. There are four substances including 4% dTT and 10% glycerol. The final concentration of DMSO was adjusted to 0.5%. The Edans/Dabcyl fluorophore–quencher pair is separated by the fluorogenic peptide. The 40 min follow up of the enzymatic reaction was done by using a Tecan Safire2 fluorimeter to observe the increase in the emission of the fluorescent dye. Enzymatic activities were estimated by calculating the slope of the linear part of the reaction curve and were normalized with respect to the activity measured in the absence of inhibitor.
A fluorescent buffer made up of 20 mM HEPES and a specific location for the cleavage site of SARS-coV-2 Mpro. NaCl is around 0.4 mM. The inhibition experiment was performed using the 4 mM DTT, 20% glycerol, pH 7.0. In the fluorescence resonance energy transfer (FRET)-based cleavage assay78, the fluorescence signal of the Edans generated as a result of the cleavage of the substrate by Mpro was observed at an emission wavelength of 460 nm with excitation at 360 nm using a Tecan Spark multimode microplate reader. The compound was diluted with 100% DMSO to prepare a stock solution. The IC50 of CIM-834 was determined after incubation of 0.5 µM of SARS-CoV-2 Mpro and CIM-834 at various concentrations from 0 to 500 µM in reaction buffer at 37 °C for 10 min. Each well at a final concentration of 50 M was then added to a final total volume of 100 l to start the reaction. The IC50 value was calculated with the help of the GraphPad Prism 9.2.0 software. Inhibitory activity of the compound was measured in triplicates and data are presented as mean ± s.d. Both positive control assays were done against Mpro.
A full-length copy of the genes of the novel SARS-CoV-2 was created by modifying the positions of 26891 and 2626 from the cell line to the artificial chromosome.
The Huh-7 cells wereseeded on the coverslips with a concentration of 8 104 cells per well. The cells were transfected with a 250 ng plasmid using the TransIT-LT1 transfection reagent, after one MCIM-834 or DMSO was added to the medium. Then, 16 h after transfection, cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature, followed by permeabilization with 0.1% Triton-X100 in PBS for 3 min at room temperature. After a blocking step with 10% normal horse serum for 15 min at room temperature, V5-tagged M proteins were visualized by incubation with monoclonal anti-V5 antibodies (Thermo Fisher Scientific, 37-7500, clone 2F11F7, 1:500) in 10% normal horse serum, followed by incubation with Alexa Fluor 488-conjugated donkey anti-mouse IgG secondary antibodies (Jackson ImmunoResearch, 715-545-151, 1:500). The trans-Golgi network was visualized using sheep anti-human TGN46 and donkey anti-sheep IgG. A cover was mounted on the Mowiol mounting medium. The images were obtained using an objective lens that was oil-immersion. The extent of colocalization between M and TGN46 was quantified by calculating the Pearson’s correlation coefficient using the JACoP plug-in of ImageJ. For both treated (1 µM CIM-834) and untreated (DMSO) conditions, 25 cells were analysed.
LigPlot 71 and UMCSAR 72 were used to calculate interactions between M and CIM-834. The mean square deviation value was obtained using the matchmaker tool. Structural-biology applications used in this project were combined with figures generated using UC Davis’ ChimeraX72.
The result was a final concentration of 44.84 M M and 100 M CIM-833, for both Fab–E and Fab–B. All components were diluted in a buffer solution comprising 20 mM HEPES-NaOH at pH 7.7, 150 mM 0.050% NaCl, 0.050% MMNG, and 0.050% CHS. Samples were mixed and incubated for 15 min on ice before vitrification. Approximately 3.5 µl of the sample was pipetted onto glow-discharged R1.2/1.3 200 mesh holey copper carbon grids (Quantifoil) and then plunge-frozen in liquid ethane using a Vitrobot mark IV (Thermo Fisher Scientific). Both datasets were collected at the Netherlands Center for Electron Nanoscopy. Grids were loaded into a Titan Krios electron microscope (Thermo Fisher Scientific) operating at 300 kV, equipped with a K3 direct electron detector and Bioquantum energy filter (Gatan). The slit width of the energy filter was set to 20 eV. Imaging was done at a nominal magnification of ×81,000 and ×105,000 for Fab–E and Fab–B, respectively, in super-resolution mode using EPU software (Thermo Fisher Scientific). A total of 5,058 and 5,037 movies were recorded for the Fab–E and Fab–B complexes, respectively. The parameters are summarized in the second table.
For tomography, semithin sections of 200 nm or 300 nm were screened and imaged using a Tecnai F30 microscope (Thermo Fisher Scientific) equipped with a Gatan OneView camera. Target positions were manually selected and acquired at ×15,500 magnification (−60° to +60° per axis; increment, 1°) by single-axis tomography (0.78 nm per pixel). The tilt series were reconstructed using IMOD56,57,58. Segmentation of selected tomograms was done manually using the brush segmentation tool in Amira-Avizo software v.2020.1 (ThermoFisher), Volume renderings and animations were also computed with the same software.
Cells prepared on sapphires for high-pressure freezing were exported from BSL3 in 6% PFA, just the same as the samples on cover. The samples were then rinsed six times with PHEM buffer (100 mM), immersed in 100 mM PHEM with 15% BSA as a cryo-protectant and high-pressure frozen using a BalTec HPM-010 with carriers forming a 40 µm-deep cavity (3-mm aluminium carriers, type B 0/0.3 mm and type 748 0.04/0.020 mm, Engineering Office M. Wohlwend). A fixative cocktail consisting of OsO4, 1% uranyl acetate and 5% water in acetone was used in the substitution of high-pressure frozen samples. The chamber temperature was increased over the course of one day, from 45 C to 50 C. Samples were then rinsed for 5 min with dry acetone on ice and further processed using a microwave, rinsed twice with ethanol for 40 s at 250 mW and infiltrated with increasing Epon 812 concentrations in ethanol (10%, 30%, 50%, 70%, 90% and 2 × 100% for 3 min each in a BioWave vacuum cycling system). At the last 100% step in Epon 812, sapphires were transferred to AFS plastic moulds and polymerized at 60 °C for 48–72 h. Ultrathin and semithin sections of 70 nm or 300 nm, respectively, were generated for both chemically fixed and high-pressure frozen samples using a UC7 Leica ultramicrotome and a 30° diamond knife (Diatome) and collected on Pioloform-coated slot grids. Grids were post-stained for 5 min with 3% uranyl acetate in 70% methanol and 2 min with lead citrate. To locate the infected cells, the Serial-EM Navigator functionality and a procedure adapted from ref. 55 were used to map the central section of a ribbon of 5 on a JEOL 1400 equipped with a TemCam-F416 camera. After the identification and storage of cells, navigator maps and grids were sent to a JEOL 2100+ which has a Matataki sCMOS camera, to view the entire perinuclear region of the selected cells.
Source: A coronavirus assembly inhibitor that targets the viral membrane protein
Animal experiments and experiments with GS-441524, VeroE6-GFP, Cherry and A549ACE2+TMPRSS2 cells
Animal housing conditions and experimental procedures were approved by the ethics committee.
GS-441524 was obtained from MedChem Express (HY-103586). Cell Signaling Technology bought Hydroxychloroquine. The person is from the Chinese city of Wuxi.
VeroE6–GFP cells (African monkey kidney cell line expressing green fluorescent protein; provided by M. van Loock, Janssen Pharmaceutica40) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% v/v heat-inactivated fetal bovine serum (FBS) A small amount of the geneticin. VeroE6–mCherry cells (generated as described in ref. 41) were maintained in DMEM, supplemented with 10% (v/v) heat-inactivated FBS and 10 μg ml−1 blasticidin. The A549ACE2+TMPRSS2 cells (a human lung carcinoma cell line overexpressing human ACE2 and human TMPRSS2 receptors), used for antiviral studies, were from InvivoGen (a549d-cov2r, A549-Dual hACE2-TMPRSS2 cells) and were cultured in DMEM supplemented with 10% v/v heat-inactivated FBS, 300 μg ml−1 hygromycin, 0.5 μg ml−1 puromycin and 10 μg ml−1 blasticidin. The A549ACE2 cells, used for subcellular studies, were generated in-house using the lentiviral technology. These cells were cultivated in DMEM supplemented with 10% (v/v) FBS, 100 U ml−1 penicillin, 10 µg ml−1 streptomycin and 1% non-essential amino acids. All assays using VeroE6–GFP, VeroE6–mCherry and A549ACE2+TMPRSS2 involving virus replication were performed in the respective cell growth medium containing 2% (instead of 10%) FBS. All cell cultures were done at 37 °C and 5% CO2. BHK-21 cells (Baby hamster kidney cell line obtained from ATCC, CCL10) were maintained in Glasgow MEM (Invitrogen) supplemented with 5% v/v FBS, 10% tryptose phosphate broth, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin and 10 mM HEPES, pH 7.4. For transfection experiments, cells were maintained in Eagle’s minimal essential medium (EMEM), as described42. 10% FBS and 1 mM Glutamax were used to cultured Huh7 cells.
A vehicle with a lot of stuff was used for the production of niematrelvit, which was formulated in a 100 and 33.3 units stock for 300 and 100 units per km, respectively. CIM-834 was formulated as 20 mg ml−1 in 14% propylene glycol (Sigma), 1% Tween 80 (Sigma), 85% pH 5 citrate buffer. To evaluate in vivo efficacy, male SCID mice (CB-17/Icr-Prkdcscid/scid/Rj; Janvier Laboratories) 7–9 weeks old were treated by oral gavage with either the vehicle (n = 12, twice a day) or CIM-834 at 100 mg per kg (n = 12 twice a day and n = 12 once a day) or nirmatrelvir at 300 mg per kg (n = 12, twice a day) or 100 mg per kg (n = 6, twice a day), starting from day 0, just before infection with the beta variant B.1.351 (hCoV-19/Belgium/rega-1920/2021; EPI_ISL_896474, 2021-01-11). Animals were inoculated with a vaccine containing 105 TCID50 and anesthetized. SARS-CoV-2 beta variant (day 0). After an initial period of 24 h, 30 h, or 48 h, the animals will be given a treatment with CIM-834, which is 100 mg per kg twice a day. The mice were kept in individually ventilated cages with three in each cage, and monitored for weight changes and clinical signs. The animals were put down by injection of 100 l Do lethal, while the lungs were collected after the third day. Infectious viral lung loads were quantified by end-point virus titration. The cells were given fresh medium after being washed to prevent carry- over of the compound during determination of the infectious virus. The cells were then put to use for three days before read-out.
All plasmids were validated by Sanger sequencing (Macrogen). There was no use of an ECM 830 Square Wave system for the preparation ofviral DNA, except for a 3 s interval between the 850 V and 0.30 ms. The electroporated cells were added to A549ACE2+TMPRSS2 cells (InvivoGen) in medium containing 10% FCS. After incubation for 6 h at 37 °C, the medium was replaced by medium with 0.2% FCS. Four days later the virus stocks were collected and then subjected to whole- genome sequencing to verify the desired sequence.
The cell lysate was put into VeroE6 cell monolayers and put into 12-well plates at 37 c for 1 h. Subsequently, the inoculum mixture was replaced with 0.8% (w/v) methylcellulose in DMEM supplemented with 2% FBS. After three days of incubation at 37 °C, the overlays were removed, the cells were fixed with 3.7% PFA and stained with 0.5% crystal violet, and plaques were counted visually.