Protonium lanthanide complexes in solution: a case study on the dynamic structure of the first sphere ligand-metal interactions
Finally, to see how their promethium complex stacked up against other lanthanide complexes, the Oak Ridge researchers combined the same ligand with all the other lanthanides. The first complete collection of comparable lanthanide complexes in solution was produced and revealed how the length of the lanthanide–oxygen bonds decreases across the lanthanide series.
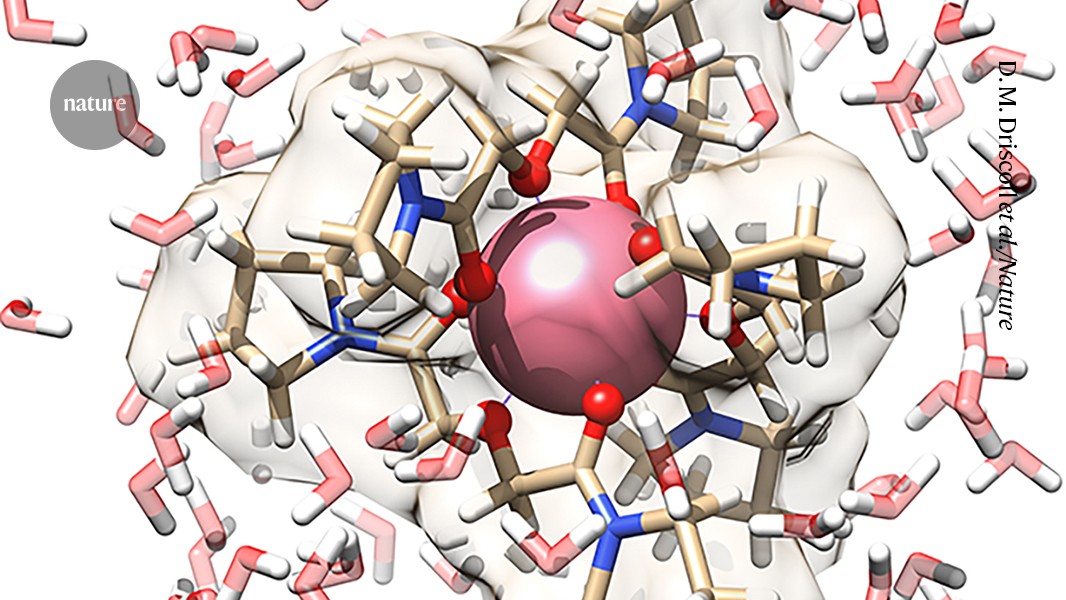
To further gain insights into the dynamic structural behaviour of PmIII complexation in an aqueous environment, we performed ab initio molecular dynamics (AIMD) simulations. The spectrum was simulation directly from the AIMD trajectory and show very good agreement with the results of the experiment. Key structural parameters can be judged based on the AIMD predicted Pm–O bond length of 2.48, as well as the analyses of radial distribution functions. The AIMD results suggest that the complex has some water structuring around it as a result of hydrogen bond interactions with the O donor groups. It is also worth noting that, like in the experimental EXAFS data, the amide carbonyl and etheric Pm–O bonds could not be resolved in the AIMD and thus appeared as a single peak in the corresponding RDF, pointing to the dynamic nature of the first-sphere ligand-metal interactions in aqueous solution (Supplementary Video 1).
A chemist at the Lawrence Berkeley National Laboratory in Berkeley, California who was not involved in the research says it is a tour de force.
Separating Promethium from Lanthanides using Coordination Complexes with Three Electron-Rich Oxide Atoms and the Elements 4f
The lanthanides are part of a group of other metals and are prized for their use in technology, including lasers and powerful magnets. Despite being abundant in Earth’s crust, rare earth elements are hard to come by and thinly spread. It is difficult to separate lanthanide elements from the rest because they share remarkably similar chemistry.
Current separation methods often use molecules known as ligands to bind to positively charged lanthanide ions in solution, forming coordination complexes. Chemists can then exploit subtle differences between these complexes to separate them: for example, by selectively washing the complexes out of water using organic solvents. “But you need lots and lots of repeated separations to get to the pure material,” says Oak Ridge chemist Ilja Popovs, who co-led the research.
Promethium has been something of a closed book for researchers working on improved separation methods. Chemists have succeeded at making only a handful of promethium compounds, all of them simple solids such an oxide3 — but never a complex that shows how promethium might bond to separation ligands in solution.
The strontium and diglycolamide were combined with three electron-rich oxygen atoms. Three of these ligands hugged each promethium ion, generating complexes with nine promethium–oxygen bonds.
The average length of bonds was measured using X-ray absorption spectroscopy and theoretical simulations. They also found that oxygen forms the bonds by providing pairs of electrons that neatly fill empty energy levels, known as orbitals, around promethium.
“It’s just incredibly difficult, skilful work, and it’s really impressive that they’ve been able to do it,” says Arnold, who studies lanthanides and their heavier cousins, the actinide elements.
With every step along the lanthanide series, from lanthanum to lutetium, each element gains one proton and one electron. Protons add to an atom’s nucleus, while electrons add to its orbitals. With lanthanides, the electrons gradually fill up a particular set of electron orbitals known as 4f that are rather diffuse and therefore do not ‘shield’ the other negatively charged electrons in an atom from the growing positive charge of its nucleus. This allows the nucleus to exert a stronger pull on some orbitals, and contract the atom more than otherwise expected.