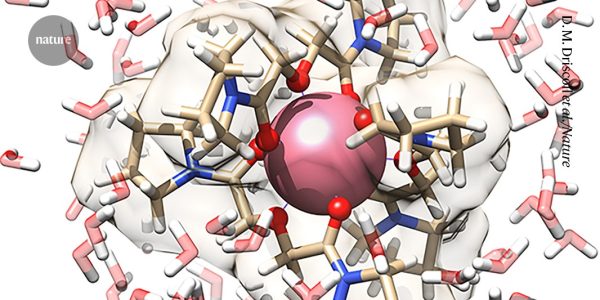
The element from the periodic table was coaxed into an elusive compound
Researchers at Oak Ridge National Laboratory have produced the first complete collection of Lanthanide complexes in solution. They found that oxygen forms the bonds byproviding pairs of electrons that fill empty energy levels around promethium. The electrons gradually fill up a particular set of electron orbitals known as 4f that are rather diffuse and therefore do not shield other negatively charged electrons in an atom.