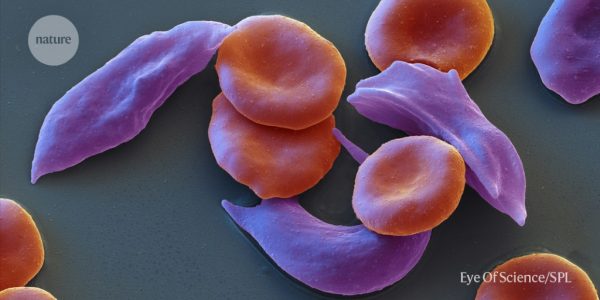
The first Crispr medicine has been approved
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has approved a therapy for patients with sickle cell disease that uses CRISPR gene editing. The therapy is called Casgevy and will be used for patients with Alpha thalassemia, a blood disorder related to sickle cell disease. In the trial, 54 participants received Casgevy and 42 patients participated for long enough to provide interim results.