The FDA approves the first treatment for a human illness
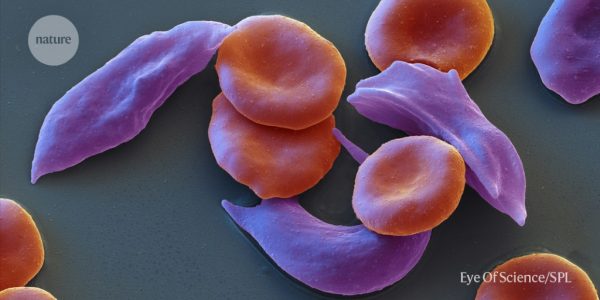
The US Food and Drug Administration (FDA) has approved two gene therapy drugs for sickle cell disease, a blood disorder where red blood cells carry oxygen through the body, to be used as treatments. One of the therapies is called ‘Casgevy’ and it uses removing cells from each patient’s bone marrow, editing a gene and then returning billions of the modified cells to patients.
The first Crispr medicine has been approved
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has approved a therapy for patients with sickle cell disease that uses CRISPR gene editing. The therapy is called Casgevy and will be used for patients with Alpha thalassemia, a blood disorder related to sickle cell disease. In the trial, 54 participants received Casgevy and 42 patients participated for long enough to provide interim results.